Bacterial Contamination of the Small Intestine as an Important Cause of Chronic Diarrhea and Abdominal Pain: Diagnosis by Breath Hydrogen Test
Geoffrey P. Davidson, MD, FRACP; Trevor A. Robb, BSc; and Chellam P. Kirubakaran, MD, DCH
From the Gastroenterology Unit, The Adelaide Children’s Hospital Inc, North Adelaide, South Australia, Australia
Abstract Unsuspected bacterial contamination of the small intestine was indicated by breath hydrogen testing in nine patients aged 2 to 34 months during physical examinations for chronic diarrhea and abdominal pain. Elevated bacterial counts of questionable significance were found in duodenal aspirates before and after antibiotic treatment. There was no evidence of bile salt deconjugation or structural changes in the small intestine by light or electron microscopy. This may indicate that the site of colonization is distal to the biopsy site. Breath testing indicated lactose malabsorption in all patients, and four of five patients tested also malabsorbed sucrose. Duodenal disaccharidase levels in all patients were within the normal ranges, but in eight patients, the lactase-sucrase ratio was greatly elevated (0.80 +/- 0.36; normal < 0.45). Dietary restriction alone did not cause complete cessation of symptoms, whereas all patients responded dramatically to oral antibiotic therapy. When patients were well, the lactase-sucrase ratio had returned to normal in those tested, and all nine had normal lactose and lactulose breath hydrogen tests. Unsuspected bacterial contamination of the small intestine, which is easily detected using the breath hydrogen test, may be more commonly associated with chronic diarrhea in children than has been previously realized. In such cases, therapy should be directed at removing the contamination. Pediatrics 1984; 74: 229-235; bacterial contamination, chronic diarrhea, abdominal pain, breath hydrogen, intestinal biopsy.
Diarrhea, abdominal pain, and malabsorption secondary to bacterial overgrowth in the small intestine, in the absence of an anatomic lesion or sever motility disorder, have only rarely been convincingly demonstrated.1, 2 This condition has been suspected as a cause of diarrhea in the pediatric age group, and there have been several reports of its association with protracted diarrhea and carbohydrate intolerance.3, 4
In the past, the diagnosis of small bowel overgrowth has relied on the culture of abnormal bacterial flora and/or demonstration of deconjugated bile salts in the intestinal fluid. Both require intestinal intubation and the results may be falsely normal due to the difficulty of culturing anaerobic organisms, the possibility of more distal colonization, the rapid absorption and dilution of free bile acids, or inadequate bacterial contact time with new bile acid secretions before aspirations.5 The advent of the breath hydrogen test (BHT) has provided a simple, rapid, and noninvasive method to detect bacterial overgrowth, especially in children.6 The use of different test sugars enables various aspects of carbohydrate malabsorption to be studied, with lactose and sucrose used to determine primary and secondary malabsorption. The unabsorbable sugar lactulose is the most suitable for detecting bacterial overgrowth because it traverses the entire bowel unabsorbed, and thus, it can titrate upper small bowel and colonic bacterial groups.
We have modified the BHT to improve its sensitivity and reliability, and we have used it as part of our investigative assessment of children with chronic diarrhea and abdominal pain.7, 8 This paper reports our findings from nine patients with a BHT suggesting bacterial overgrowth.

PATIENTS AND METHODS
All nine patients were referred to the Gastroenterology Unit of the Adelaide Children’s Hospital. The age range and symptoms at examination are shown in Table 1. All children except one had four to seven sloppy, often offensive, bowel actions daily. The child with abdominal pain had a normal bowel habit. Because of lack of associated growth failure, it was felt that these children had a chronic, non-specific diarrhea. No evidence of steatorrhea was noted on stool microscopy findings. There were no associated diseases except in patient 8 who had cystic fibrosis. The infant with bloody diarrhea had normal stools prior to the onset of symptoms. A rectal biopsy excluded Hirschsprung’s disease. Control subjects for the BHT were ten children of hospital staff members who had no gastrointestinal symptoms at the time of testing. The mean age of 36 months was higher for the control children than for the patients.
Stool specimens were cultured for bacteria and viruses and examined by electron microscopy. Sweat tests were performed on four patients; findings were positive only in patient 8. Duodenal intubation and biopsy were carried out on all patients following sedation with quinalbarbitone and using metaclopramide to increase gastric emptying and upper gastrointestinal motility. A sterile No. 8 French feeding tube was attached to the intestinal biopsy capsule tubing, and duodenal juice of PH7 or greater was collected on ice for aerobic and anaerobic bacterial culture and measurement of bile acids. The average time taken for the biopsy and juice collection was 15 minutes. Routine culturing was done on horse blood agar (incubated in 5% CO2), and anaerobic blood agar incubated under anaerobic conditions. For colony counting, cultures were inoculated onto horse blood agar plates, incubated in 5% CO2 for two days, and then read according to the method of Miles et al.9 Duodenal mucosa was obtained using a Watson pediatric biopsy capsule as described by Townley and Barnes, 10 and was divided for histology, disaccharidase estimation, and electron microscopy. When assessing the results of microbial culture, the level considered by ourselves and others as abnormally high at the site samples was > 104 colonies per milliliter.11-15
Our method of hydrogen (H2) collection and analysis procedure has been described previously7. An important aspect of the methodology is sample quality correction of H2 values based on expired oxygen levels. By correction to a common oxygen level, H2 responses between individuals can be quantitatively compared. Without such a normalization procedure, small H2 changes and early transient peak H2 productions tend to be lost in the highly variable values obtained with various quality breath samples. This is especially so in children in whom cooperation and constancy of breath samples is not always possible.
The lactose BHT used 2 g/kg of lactose (maximum 20g) in 100 mL of water with 30-minute interval sampling. The sucrose BHT used 30 g of sucrose in 100mL of water, a level we have found is normally absorbed readily even in young infants.
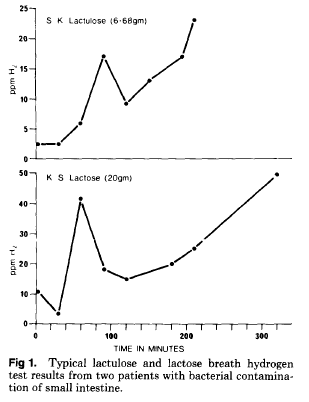
Bacterial overgrowth was indicated by an early transient H2 increase preceding a later H2 increase when unabsorbed carbohydrate reached the colon (Fig. 1). The magnitude of the H2 responses was unimportant, as only the characteristic double peak was used to detect patients with suspected overgrowth. In most patients, the early transient H2 increase was first noted during a routine lactose BHT, and the suggestive overgrowth result was then demonstrated the next day by a second BHT using unabsorbable lactulose (10mL of Duphalac syrup) in 100 mL of water. Samples were collected every 20 minutes for the first 60 minutes, and at 30-minute intervals thereafter with lactulose testing.
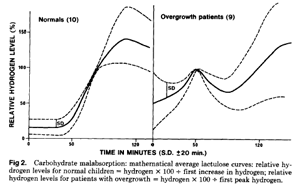
RESULTS
Electron microscopy and culture of stools failed to detect pathogenic organisms in any child. Findings from a barium meal and follow-through examination were normal in all but two children (patients 7 and 9) in whom the features were entirely nonspecific but suggested a pattern consistent with malabsorption.
Bacterial culture of duodenal aspirate yielded a variety of microorganisms as shown in Table 1. The organisms most commonly isolated were of an oral type19, i.e., they were species regarded as normal flora and included streptococci of the viridans group, Staphylococcus albus, Staphylococcus aureus, Lactobacillus sp (aerobic and anaerobic), and Diplococcus pneumoniae. Organisms regarded as being of fecal type were isolated in numbers > 104 organisms per milliliter which is a significant level of growth in the upper small intestine.11-15 Oral-type flora alone were cultured from only one patient; the remaining patients had a mixture of both oral and fecal flora.
Deconjugated bile acids were not detected in duodenal juice from any patient.
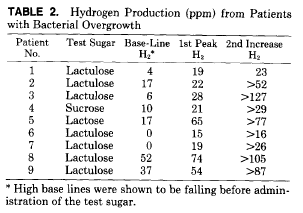
The duodenal mucosal architecture as viewed by both light and electron microscopy was normal in all patients. Disaccharidase levels for lactase and sucrase and the corresponding lactase-sucrase ratios are shown in Table 1. All children had normal lactase levels, but the BHT indicated lactase malabsorption and bacterial overgrowth, the fast H2 level was elevated (Table 2). A base-line sample 30 minutes later showed a decrease in all cases. This is in keeping with our previous experience that elevated fasting levels continue to decrease to normal levels over time. Patients 2 and 8 had low sucrase levels, but only patient 8 was shown to malabsorb sucrose by BHT. Patients 4, 7, and 9, who had normal sucrase levels, were show to malabsorb sucrose.
The lactase-sucrase ratios were greater than normal in all but one patient (mean +/- SD 0.80 +/- 0.36), and were significantly different from those of 16 normal subjects (0.40 +/- 0.16 P < .01) and seven obligate heterozygotes for sucrase-isomaltase deficiency (1.33 +/- 0.34). Mean lactase levels in patients with bacterial overgrowth (45+/- 30) were not significantly different from those of control patients (37 +/- 22). Mean sucrase levels in patients with overgrowth (64+/- 41), although appearing lower than those of control patients (91 +/- 42), were not significantly different.
Initial treatment in these children was dietary modification by lactose withdrawal in all patients and added sucrose withdrawal when indicated by BHT. Because of incomplete resolution of symptoms in all patients, oral antibiotic therapy was tried as outlined in Table 1. The response to this treatment was often dramatic, leading to complete resolution of symptoms within 24 hours. The antibiotics were chosen according to the sensitivity of the organisms cultured from duodenal juice except in three patients (patients 5, 8, and 9) who were given antibiotics for other indications, otitis media, pneumonia, and colitis, respectively. All children were able to tolerate disaccharides after antibiotic treatment with no evidence of malabsorption either clinically or by BHT. Lactulose BHT levels returned to normal in all children after antibiotic therapy and showed a normal colonic H2 increase from the unabsorbed carbohydrate without the early transient increase seen prior to treatment.
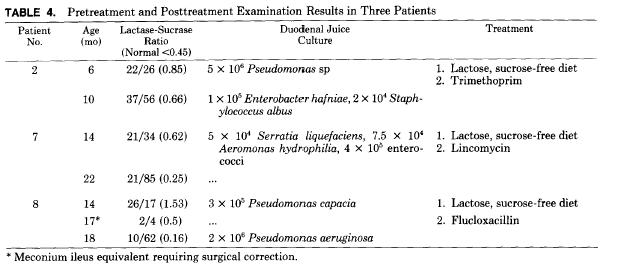
Three children had their biopsies repeated when they were asymptomatic and the BHT level had returned to normal (Table 4). The lactase-sucrase ratios had altered, sucrase levels had increased, but significant bacterial colonies were still present in the two duodenal juice samples cultured. Patient 8 had a lower lactase level after treatment due to the development of meconium ileus equivalent, which markedly lowered all disaccharidase levels before surgical correction.
DISCUSSION
The children in this study were examined because of chronic diarrhea or abdominal pain. The diagnosis of bacterial contamination of the small intestine was not previously suspected and may have been undetected without the use of the BHT. In four children the fasting breath H2 level was elevated; this is an unusual finding and may be a useful indicator of the presence of bacterial overgrowth.
The finding of equally elevated bacterial counts after treatment in the two patients studied probably reflects the inadequacy of the sampling site in that pathogenic organisms may have been present more distally. The presence of both oral- and fecal-type flora indicates that some of the contamination was due to organisms passing into the upper intestine during intubation. The lack of clearance of the organisms after antibiotic therapy in the face of obvious clinical improvement would seem to make it unlikely that these organisms were the cause of the diarrhea illness.
Using sensitive methodology, we were unable to detect the presence of deconjugated bile salts in the duodenum in any patient. Our findings do not support the suggestions of Gracey et al, 20-21 Challacombe et al, 22 and Kilby et al23 who wee also unable to find evidence of bile salt deconjugation in patients with carbohydrate intolerance. It is possible that the sampling site influence our findings and that deconjugated bile salts may have been found more distally. Rapid absorption of free bile acids, luminal dilution below detection limits, or inadequate bacterial contact time for new secretions prior to aspiration may have prohibited our finding free bile acids.5
The duodenal mucosa was normal in each patient as was the anatomy of the small intestine as determined by barium studies in the majority of children. Gracey et al24 had suggested that ultra structural changes detected in rats with blind loops may be specific for the condition. We feel, however, that these structural changes may be artifactual as we found them to be present both before and after successful therapy.
The duodenal mucosal disaccharidase levels in our patients were well within the normal range except for sucrase depression in two patients. Despite this, all children showed biochemical evidence of lactose malabsorption by the BHT, and four of five showed malabsorbed sucrose by breath testing. We believe this demonstrates the increased sensitivity of the BHT compared with the measurement of disaccharidase levels. The BHT measures the ability of the whole small intestine to absorb carbohydrate, whereas the duodenal biopsy samples only a minute area of intestine.25
An unusual finding and one that is difficult to explain is the alteration of the lactase-sucrase ratio which differed significantly from that of normal children and also from obligate heterozygotes for sucrase-isomaltase deficiency. It is probable that the altered ratio is directly related to bacterial contamination because the ratio returned to normal in three patients when well. An alteration of the lactase-sucrase ratio may be an indicator of bacterial colonization of the upper intestine.
We believe this study demonstrates the value of the BHT in the diagnosis of bacterial contamination in the small intestine of children. The BHT is simple, rapid, noninvasive, and removes the necessity for duodenal intubation or anaerobic culture facilities to make the diagnosis. We have not tested for other parameters thought to reflect bacterial overgrowth, e.g., Schilling test or fat absorption, due to their reported negligible predictive value for detecting patients with high colony counts.26
It seems likely that bacterial contamination played an important role in the pathogenesis of the diarrhea in these children. After finding biochemical evidence sucrose and/or lactose malabsorption and the bacterial overgrowth, dietary manipulation alone was tried with partial improvement but without totally relieving symptoms. However, antibiotic therapy brought immediate relief in all patients, suggesting that sterilization of the upper intestine was an important factor in bringing about clinical recovery. In all patients, the BHT results for lactose and lactulose returned to normal when the patients were well. It seems unlikely that the symptoms resolved spontaneously as these children had been symptomatic for many weeks prior to treatment. The cause of the bacterial contamination in these previously healthy children remains obscure.
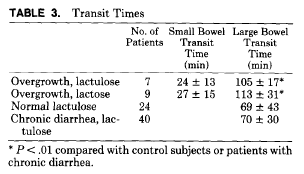
We did note that the transit time from mouth to cecum was significantly increased in this group compared with values for normal children or other children with diarrhea. It may be that there is some degree of stasis and this, together with an initiating insult, allows the establishment of an abnormal flora which then causes symptoms to persist.3 Coello-Ramirez et al4 have suggested that proliferation of bacteria within the small bowel of infants with diarrhea may be related to the presence of undigested carbohydrates that were present in our patients. It is possible that bacterial overgrowth may be the reason for continuing symptoms and may represent one of the causes or consequences of chronic diarrhea. Hopefully, our findings will increase the suspicion of bacterial overgrowth in young children and infants with chronic diarrhea who have been unresponsive to dietary carbohydrate restriction.
ACKNOWLEDGEMENTS
We thank A. Lawrence, Department of Microbiology, the Adelaide Children’s Hospital, for bacterial cultures; Dr. G Bunn, Department of Histopathology, the Adelaide Children’s Hospital, for light and electron microscopic studies; and G Hill, Department of Chemical Pathology, the Adelaide Children’s Hospital, for the disaccharidase estimations. We also thank the pediatric consultant staff for allowing us to study patients under their care, J. Walker for typing the manuscript, and C. Lloyd, medical illustrator, and the Clinical Photography Department, the Adelaide Children’s Hospital, for production of figures.
REFERENCES
-
Roberts SH, James O, Jarvis EH: Bacterial overgrowth syndrome without ‘blind loop’: A cause for malnutrition in the elderly. Lancet 1977; 1193
-
Ruddell WSJ, Losowsky MS: Severe diarrhea due to small intestinal colonization during cimetidine treatment. Br Med J 1980; 2: 273
-
Challacombe DN, Richardson JM, Rowe B, et al: Bacterial micro flora of the upper gastrointestinal tract in infants with protracted diarrhea. Arch Dis Child 1974; 49: 270
-
Coello-Ramirez P, Lifshitz F, Zuniga V: Enteric micro flora and carbohydrate intolerance in infants with diarrhea. Pediatrics 1972; 49: 233
-
Egger G, Kessler JI: Clinical experience with a simple test for the detection of bacterial deconjugation of bile salts and he site and extent of bacterial overgrowth in the small intestine. Gastroenterology 1973; 64: 545
-
Rhodes JM, Jewell DP, Middleton P: The lactulose hydrogen breath test as a diagnostic test for small-bowel bacterial overgrowth. Scand J Gastroenterol 1979; 14: 333
-
Robb TA, Davidson GP: Advances in breath hydrogen quantitation in paediatrics: Sample collection and normalization to constant oxygen and nitrogen levels. Clin Chim Acta 1981; 111: 281
-
Davidson GP, Robb TA: The value of breath hydrogen test in the management of diarrhoeal illness in children. Aust Pediatr J 1980; 16: 133
-
Miles AA, Misra SS, Irwin JO: Estimation of bactericidal power of blood. J Hyg Camb 1939; 38: 732
-
Townley RRW, Barnes GL: Intestinal biopsy in childhood. Arch Dis Child 1973; 48: 480
-
Dellipiani AW, Girdwood RH: Bacterial changes in the small intestine in malabsorptive states and pernicious anemia. Clin Sci 1964; 26: 359
-
Barnes GL, Bishop RF, Townley RRW: Microbial flora and disaccaridase depression in infantile gastroenteritis. Acta Pediatr Scand 1974; 63: 423
-
Gracey M, Suharjono, Sunoto, et al: Microbial contamination of the gut: Another feature of malnutrition. Am J Clin Nutr 1973l 26: 1170
-
Rogers AI, Rothman SL: Blind loop syndrome. Postgrad med 1974; 55: 99
-
Broido PW, Gorbach SL, Nyhus LM: Micro flora of the gastrointestinal tract and the surgical malabsorption syndromes. Surg Gynecol Obstet 1972; 135: 449
-
Robb TA, Davidson GP: Analysis of individual bile acids and their glycine taurine conjugates by high performance thin layer chromatography and densitometry. Ann Clin Biochem 1984; 21: 137
-
Dahlqvist A: Method of assay of intestinal disaccharidases. Anal Biochem 1964; 21: 137
-
Kerry KR, Townley RRW: Genetic aspects of intestinal sucrase-isomaltase deficiency. Aust Paediatr J 1965; 1: 223
-
Bishop RF, Barnes GL, Townley RRW: Microbial flora of the stomach and small intestine in infantile gastroenteritis. Acta Paediatr Scand 1974; 63: 418
-
Gracey M, Burke V, Anderson CM: Association of monosaccharide malabsorption with abnormal small intestine flora. Lancet 1969; 2: 384
-
Gracey M, Burke V, Oshin A: Reversible inhibition of intestinal active sugar transport by deconjugated bile salts in vitro. Biochim Biophys Acta 1971; 225: 308
-
Challacombe DN, Richardson JM, Edkins S: Anaerobic bacteria and deconjugated bile salts in the upper small intestine of infants with gastrointestinal disorders. Acta Pediatr Scand 1974; 63: 581
-
Kilby AM, Dolby JM, Honour P, et al: Duodenal bacterial flora in early stages of transient monosaccharide intolerance in infants. Arch Did Child 1977; 53: 228
-
Gracey M, Papadimitriou J, Bower G: Ultrastructural changes in the small intestines of rats with self filling blind loops. Gastroenterology 1974; 67: 646
-
Harrison M, Walker-Smith JA: Reinvestigation of lactose intolerant children: Lack of correlation between continuing lactose intolerance and small intestinal morphology, disaccharidase activity, and lactose tolerance tests. Gut 1977; 18: 48.
-
Mayer PJ, Beeken WL: The role of urinary indicant as a predictor of bacterial colonization in the human jejunum. Am J Dig Dis 1975; 20: 1003